Abstract
Introduction
Natural killer (NK) cells contribute to the immune surveillance of cancer and are capable of controlling acute myeloid leukemia (AML) in vitro and following allogeneic hematopoietic stem cell transplantation. NK cell activation is dependent on the balance of activating and inhibitory receptor-ligand interactions. The prognostic impact of the expression of NK receptor ligands (NKRL) on AML blasts at diagnosis has not been described. We investigated the impact of NKRL expression by AML on the outcome of newly diagnosed patients undergoing induction chemotherapy. We hypothesized that patients with AML that expressed NKRL that facilitated NK cell activation (low inhibitory and high activating ligand expression) have improved immune surveillance and a reduced relapse rate.
Methods
Cryopreserved bone marrow cells and clinical data of 66 AML patients who underwent induction chemotherapy between 2001 and 2013 were obtained from the Australasian Leukemia Lymphoma Group tissue bank. Patients were stratified by cytogenetics (CG) and mutation status (European LeukemiaNet criteria, ELN). Expression (by relative fluorescence intensity - RFI) of 6 activating (MICA, MICAB, CD155, CD112, ULBP1, ULBP2/5/6) and 3 inhibitory (HLA class I, PD-L1 and PD-L2) NKRL on AML blasts was analyzed by flow cytometry. A net score of activating minus inhibitory ligands was calculated, which stratified patients into 2 groups: G0 (inhibitory pattern) and G1 (activating pattern).
Results
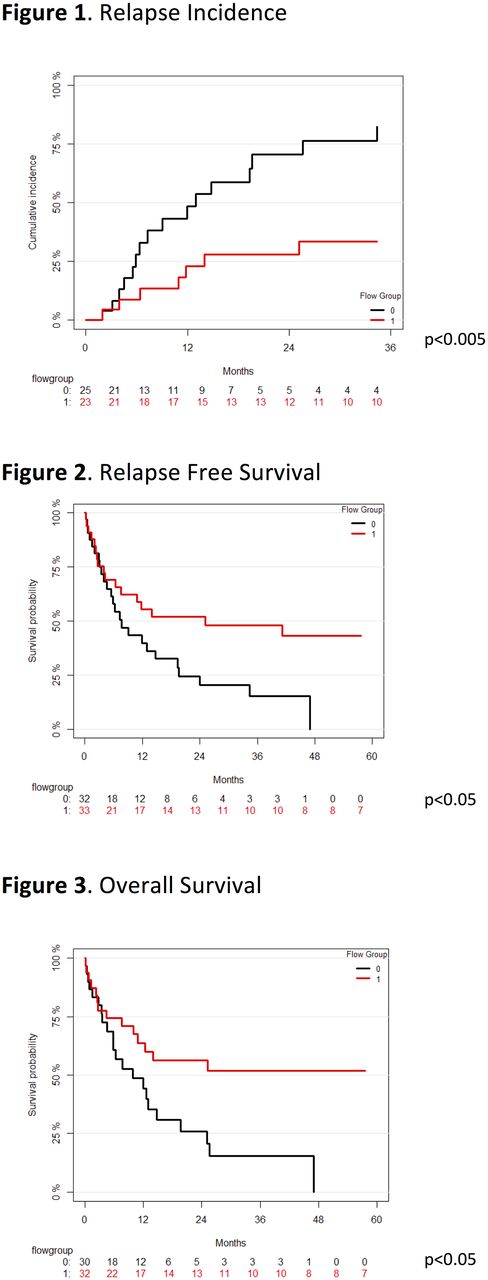
The median age of patients was 56 years. Cytogenetic risk was adverse (13), intermediate (46) and favorable (7); ELN risk incorporating mutational profile was adverse (25), intermediate (15), and favorable (20). 79% of patients received an anthracycline in combination with cytarabine as induction therapy. Among activating ligands, the MICA, CD112 and ULBP1 were frequently expressed by AML blasts (79%, 80% and 82% of patients respectively), and also demonstrated the greatest intensity of expression (mean RFI of 3, 4.2 and 6.5 respectively). When analyzed individually, only ULBP1 expression was significantly associated with improved overall survival (OS) (p<0.05), relapse free survival (RFS) (p<0.05) and lower relapse incidence (RI) (p<0.005). The intensity of ULBP1 expression was significantly associated with improved OS, RFS and RI in a stepwise fashion. Patients with the highest ULBP1 expression had a reduced hazard ratio of death (HR 0.20, p<0.05) or relapse (HR 0.24, p<0.05) compared with non-expressors. PD-L2 expression on AML cells was higher and more frequent than PD-L1 (85% vs 5%). All AML variably expressed HLA class I. According to RFI, 32 patients were assigned to G0 and 34 to G1. There was no difference in patient age or distribution of CG or ELN risk categories between patients in G0 or G1 subgroups. G1 was significantly associated with lower relapse incidence than G0 (p<0.005, 2-year-RI: G1 28% vs G0 71%, Fig. 1). G1 was associated with higher RFS (log-rank p<0.05; at 2 years: G1 52% vs G0 20%; Fig 2) and improved OS (log rank p<0.05; at 2 years: G1 56% vs G0 29%; Fig 3). The G1 phenotype remained significantly associated with lower RI when taking into account either CG or ELN risk.
Conclusions
We have identified an immunological risk stratification method based on NKRL expression which is independent of established cytogenetic and molecular prognostic factors. Expression of activating NK cell receptor ligands by AML blasts is associated with superior survival and reduced relapse following induction chemotherapy. Our findings suggest that AML blasts are effective targets for NK cell-mediated immune surveillance, and support investigation into therapeutic strategies to enhance NK cell activation as a means of maintaining remission.
Ritchie: Amgen Inc.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal